During a dinner conversation with a colleague last week, I was reflecting on why clinical trials were so complicated.
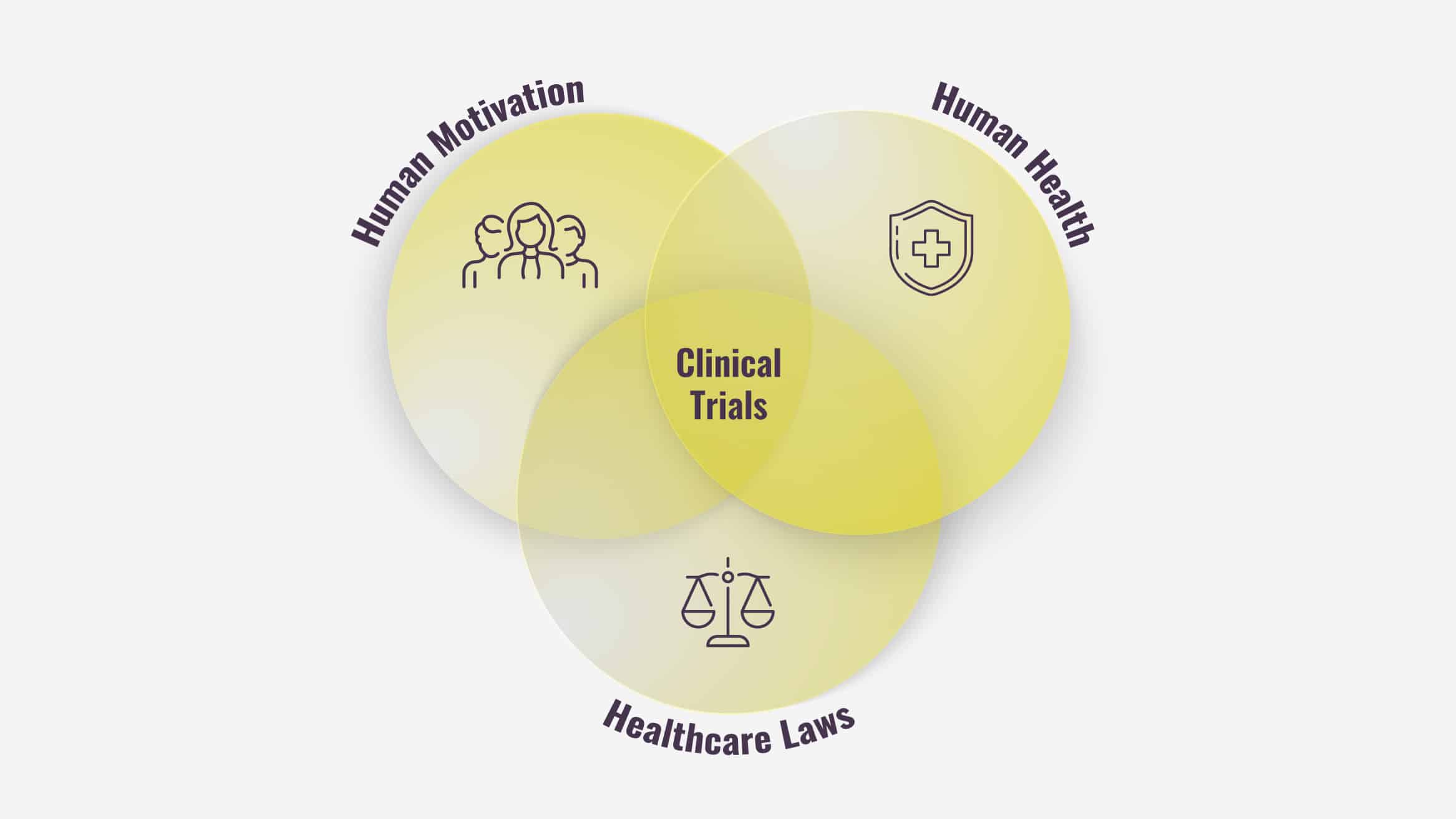
It all boils down to three reasons:
Human Motivation
Many of us (including myself) underestimate human psychology and motivation.
At an individual level, each of us is motivated by different things (status, money, freedom, etc.)
At a macro level, private for-profit organizations want to maximize profits (think CROs, biopharmaceutical and medical devices companies), non-profit organizations want to achieve their mission (think large academic sites), or the government (think US Food and Drug Administration) want patients to be safe.
When there is a mismatch between individual and institutional motivation, project planning and execution becomes difficult. There is a delicate dance going on between individual and institutional motivations.
When we conduct clinical trials, we cannot ignore human motivation.
Human Health
When we’re sick, the only thing that matters is improving our health and feeling better.
Clinical trials can provide human subjects with hope for better health. But hopes don’t always turn into reality. A patient’s health may improve, may stay the same, or in some cases worsen. We just don’t know.
In routine clinical care where doctors perform their duties under medical guidelines using commercially available medical products. There is clear evidence on what works and what doesn’t.
When we’re conducting clinical trials, we don’t know the impact of our work on human health. This uncertainty can be painful and frustrating.
Healthcare Laws
Healthcare laws, specifically clinical trial laws and regulations, reside at the intersection of medical innovation and human safety.
In other words, the government has enacted laws to protect clinical trial participants from unwanted side effects of medical innovation such as a new drug or medical device.
The hardest part of conducting clinical trials within any legal framework are as follows:
- Laws keep changing with changes in technology (for example, medical products built with artificial intelligence and machine learning algorithms require new set of regulations)
- Every country, state, and city have their own legal structure which can be difficult to navigate, (for example, in the State of California, patients not only review and sign the research informed consent, but are also required to review and sign Experimental Bill of Rights when participating in a clinical trial), and
- Medical Standard of Care can vary within and across geographies. In other words, treatment protocols can vary from one country to another or even one hospital system to another.
In summary, human motivation, human health, and healthcare laws make clinical trials a complicated endeavor to pursue and manage.
If you can master one or more of these three forces at play, you can have a positive impact on clinical trial design and conduct.

1 Thought on "Why Are Clinical Trials Complicated?"
Lorelle Silveira
February 20, 2024Hi Kunal, research does show that equally motivating for Clinical Trial participation (along with serious health condition and to improve health outcome) is an altruistic one to help others with the same condition/disease etc. And research also shows CT recipients report better quality of life because they have closer relationships with clinicians and their health care team and treatment of care they receive. And that’s for successful and non-successful participants!