To learn more about site reads and the role of medical imaging software, I invited Kelie Williams Luby, Vice President of Clinical Trials at Mint Medical.
Kelie has 24 years of experience as a clinical trial researcher, first as a medicinal chemist, and for the past 19 years as a clinical trialist in medical imaging-based endpoints.
She is passionate about optimizing clinical trial designs while reducing the cognitive burden placed on healthcare providers treating patients in those clinical trials.
Kelie believes it is possible to improve the efficiency of how clinical trials with imaging-based endpoints are conducted while also improving the reliability of data abstracted from participants during clinical trials.
She holds a B.S and M.S in Chemistry along with a Master’s in Technical, Scientific, and Medical Communication.
Please join me in welcoming Kelie on the Clinical Trial Podcast.
Sponsor:
This podcast is brought to you by Calyx. Calyx is a trusted name in medical imaging, having delivered imaging services to meet the needs of global biopharmaceutical sponsors and clinical research organizations for over 25 years. To learn more, visit https://www.calyx.ai/
We put a lot into the EDC, we put a lot into how we’re going to get data away from the site. But how much technology are we invested in helping the sites in their day to day and actually doing the work that we’re asking them to do so that we can get that data? – Kelie Williams Luby
Selected Links From The Episode:
Connect with Kelie Luby Williams – LinkedIn
WHO Criteria – Reporting Results for Cancer Treatment (1981)
Clinton-Kessler Oncology Initiative
Transcelerate Biopharma Common Protocol Template
Association of Clinical Research Professionals (ACRP) Training Catalog – iRECIST training can be found here and here.
iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics
Mint Medical imaging software
Picture archiving and communication system (PACS)
Prostate Imaging Reporting & Data System (PI-RADS)
Lung CT Screening Reporting & Data System (Lung-RADS®)
Musculoskeletal Reporting and Data System (MSK-RADS)™
Prostate Cancer Working Group 3 (PCWG3)
Made to Stick: Why Some Ideas Survive and Others Die by Charles Kahlenberg, Chip Heath, et al.
A Seat at the Table: IT Leadership in the Age of Agility by Mark Schwartz
Show Notes:
[3:42] Imaging workflow at a site in a typical oncology trial
- Site enrolls patients
- Patient undergoes protocol specific imaging scans and tumor response assessments such as a RECIST 1.1 assessment
- Patient undergoes standard of care (SOC) imaging scans and radiology routine evaluations. This information goes into a Tumor Response Form
- Both protocol and standard of care radiology assessments are shared with (a) PI/ oncologist, and (b) the trial sponsor via electronic data capture (EDC)
This information helps determine to determine whether the patient will stay on study, what the status of their disease course is, and how they carry on throughout the remainder of the trial, and they’re scheduled to them
[7:32] The standard of care and protocol requirement reads may differ
[8:08] Interpretation of the protocol requirements and response criteria can vary from site to site.
- There are a handful of response criteria which may be protocol specific, treatment specific, reiterations of prior versions, etc.
- Protocol need more specificity, more details for the end user in terms of how they’re applying a particular protocol, specification for evaluation
[12:41] Ideal working relationship between oncologist and radiologist at a site
- Information is being assessed by the radiologist and then sent to the oncologist
- Oncologist is then interpreting the structured reported information from the radiologist in order to make a decision on the patient
[14:01] History of response criteria
- Clinton-Kessler Oncology Initiative of 1996 – we can have imaging as a surrogate endpoint
- FDA Modernization Act of 1997 – we can do multiple endpoints on a clinical trial
- WHO Criteria
- RECIST 1.0
- RECIST 1.1
Now we have response criteria for different response criteria for different indications. There is response criteria for non-imaging indications or evaluations
[18:14] Site read versus central read
- Site read
- Evaluation of the patient at the site of how they’re doing on the treatment
- A way for the data to lead the site through the EDC, so it can go back to the Sponsor, and the Sponsor can then evaluate how their therapy is working
- Central read
- It’s about variability, bias, and our trust in the data
- Additional analysis that needs to be done on the imaging data that perhaps would not be appropriate or burdensome for the site to do
- Collection of all of the scans from the various clinical sites, and then doing an objective review of that imaging data
- Involves trained physician radiologists readers that are guided by an imaging charter or by a reader rules manual on how to approach the read
- Controlling the workflow in terms of sequential locked review
[21:04] Discrepancy between site read and central read i.e. site-central read discordance and how important is it?
- Depends on the endpoint of the study
- How information is collected and reported
- Indication in question
[22:05] Why do we have site-central read discordance?
- Imaging CROs have software tools that they use to do the analysis. Sites don’t always have that
- Sites may be using a paper form
- Radiology transcription and EDC data entry procedure
[22:37] Are sites that are not using imaging software at a disadvantage?
- It is beneficial for sponsors to have sites that do have imaging software that can help them and assist the radiologists and the PI in doing study evaluations
[22:39] Are site reads and central reads assessed by the same or different criteria?
- Both reads are assessed by the same criteria based on the protocol, which serves as the guiding document
- Imaging CRO develops an imaging charter that may refine the assessment – what do in corner cases? What to do in gap cases?
[25:28] “Compare to Prior” issue
- Results stable compared to prior scan versus baseline
- Benefit of response evaluations because they compare to baseline
[26:38] Training plan for a radiologist participating in clinical trial versus radiologist responsible for routine clinical care
- Training is limited/ lacking at the site level
[27:49] Should sites look at what the case report forms require them to enter and then work backwards to see if their workflow matches that?
Issue sites face:
- Sites contact the imaging CRO/ lab about about a protocol required assessment
- Imaging CRO/ lab look at the text heavy protocol together
- Imaging CRO/ lab advises the site to look at the EDC guidelines for data collection/ entry
- After trying to figure out how to get and report the data, the site and imaging CRO/ lab end up back to the protocol
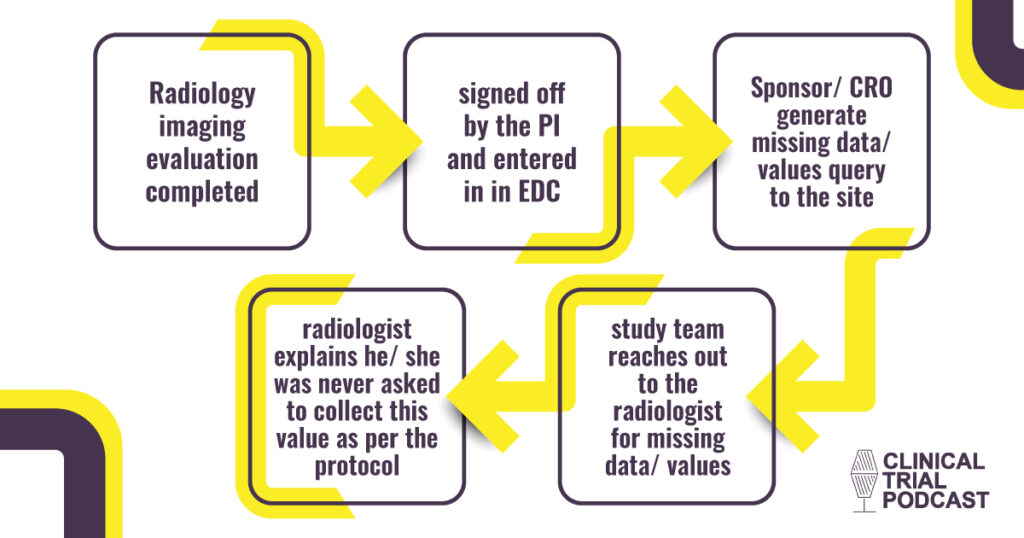
Radiology imaging evaluation completed → signed off by the PI and entered in in EDC → Sponsor/ CRO generate missing data/ values query to the site → study team reaches out to the radiologist for missing data/ values → radiologist explains he/ she was never asked to collect this value as per the protocol
This increases the cognitive burden on the sites and it takes time away from treating patients.
Proposed solution:
- Everything that is needed from the data collection should be in the protocol…nothing should live outside of the protocol
- We need think about how we’re capturing the data in the end
- Consider timing of when the protocol is finalized in relation to the EDC
- Road test your case report forms – go through the process of what that looks like
[32:53] Downside of being too specific in the protocol and how it leads to protocol amendments
[33:48] Get research coordinators at 2-3 sites review and critique your protocol and case report forms
[34:38] Process to educate yourself on an imaging criteria – learn to bucket the different assessments that are being required per protocol
- What is the indication?
- What is qualitative in the evaluation?
- What is the quantitative in the evaluation?
- What are the measurements that are required to do the quantitative evaluation?
[38:09] ***Sponsor Message***
Calyx is a trusted name in medical imaging.
Calyx’s experience includes over 2,600 clinical trials and 270 FDA supported approvals and spans multiple therapeutic areas, with significant concentration in oncology and CNS-related drug development across all phases, geographic areas, and degrees of complexity.
To learn more about how Calyx can help you with your imaging needs, visit calyx.ai or contact your Calyx solutions specialist today.
[39:47] Benefits for utilizing medical imaging software
- Time savings – the medical imaging software is assisting/ guiding you through the analysis that you’re performing
- Structured data reporting
- Maximizing the data that we can abstract from an image
- Keeping the information from imaging data intact digitally for the patient as it moves through the process – data liquidity
[42:04] Alternatives to imaging software – paper forms
[43:47] Downsides of measuring or performing tumor measurements directly in PACS
- PACS is for viewing and moving imaging information
- PACS is not intended for capturing information
[44:57] Imaging assessment process or criteria between protocols and routine care
- There are published guidelines for screening and staging evaluation
- Response criteria is a communication language. It’s a way to communicate information and structure and to make it comparable
[46:12] What is screening and staging?
- Patients are screened based on assessment tools such as PI-RADS, Lung-RADs etc.
- Patients are staged based on the status of where the disease is, how it’s confined, if it’s metastasized, if you have lymph nodes, metastatic sites etc.
- Staging is part of a cancer study protocol inclusion and exclusion eligibility criteria
- Criteria such as RECIST are intended to evaluate for structured data reporting
- There are separate guidelines for therapy evaluation
[49:41] Protocol specific evaluation criteria i.e. modified criteria
- Based on a particular drug mechanism of action
- Sponsor is looking to evaluate the particular treatment effect or otherwise
- A patient population specific modification may be necessary
- Sometimes it’s not clear in the protocol what aspect of the criteria has been modified.
- Sites don’t want to have to go to the end of the line, when they’re reporting data to figure out what they should have done at the beginning
[55:59] How to road test a clinical trial protocol before finalizing it?
- Have a research coordinate at one or more sites review and critique your draft protocol
- Do a sample case
- Does the end user have the tools at their site to report what they’re being asked to do per protocol
Good, comprehensive medical writing is the glue that holds together all the documents
[58:11] Based on the protocol, ask the sites if they have the means to do the evaluations
[59:01] Is there integration between imaging software and electronic data capture (EDC)?
- It’s possible to go from site radiology read straight into EDC. We don’t always do that and it involves manual data entry
- Importance of automation – site imaging assessment and sponsor EDC integration
[1:08:55] Sites using their own imaging software – Bring Your Own Software (BYOS)
[1:11:48] Imaging challenges when a patient is participating in multiple clinical trials
[1:12:37] Challenges when different hospitals or different technicians within the same hospital capture images
[1:13:06] Are sites still using X-Ray films or are imaging scans now digital?
[1:13:35] Interactive lesion location and machine readable imaging data
[1:15:00] Medical writing skills
- You learn how to read a protocol
- When you write things, but you don’t actually know how they work in the context of real use
- You have to try to get more information on how it’s done. Once you see it, and you understand it, then the context is linked, and that really sticks
- If there is an indication that you’ve never worked on before, you have to go and find patient and people stories. You have to understand what the actual human context is, what the patient experiences and what the provider experiences treating that particular indication. This will help tie all together with what your writing on
[1:17:23] Challenges as a medical writer
- Not understanding the context of what you’re writing about
- Not seeing what the data looks like on the other side
[1:20:56] ***Sponsor Message***
Calyx has been a trusted name in medical imaging for almost two decades.
Kunal shares his own personal experience working at Perceptive Informatics (now Calyx).
To learn more about how Calyx can help you with your imaging needs, visit calyx.ai or contact your Calyx solutions specialist today.
Why We Have Missing Medical Imaging Data?