Almost everyone I’ve met in clinical research has accidentally discovered this hidden profession.
Your interest in science and medicine somehow got you involved in clinical research and clinical trial management.
But didn’t you wish you had a crystal clear understanding of “Who’s Who In Clinical Research?”
That’s when this post comes handy.
Clinical Research is sometimes also referred to as Medical Affairs or Clinical Affairs.
Aside from the naming nuances, the pillars of clinical research remain the same. This is true for pharmaceuticals, medical devices, and biologics.
In this post, you’ll learn the functional areas that form an ideal clinical research team at a sponsor or clinical research organization (CRO).
For each function, I’ve highlighted why the group exists and their general mindset.
We’ll wrap up our discussion with adjacent departments that interact with clinical research professionals.
So let’s get started:
Clinical Project Management
I’d like to start with my personal favorite clinical research role, Project Management.
The reason why the project management role is so interesting is because it provides a holistic view of clinical trial management and clinical research.
“General Contractor” for the study
The project management team is made up of Clinical Project Managers and Program Managers.
In some companies, Clinical Project Managers (CPM) may be referred to as Clinical Trial Managers (CTM) or Study Managers.
Project managers are the “General Contractors” of clinical research. They are accountable of all aspects of a clinical trial.
Go-to person for clinical trial budget, timelines, resources
Developing and actively managing clinical trial finances, timelines, and resource allocation is necessary for a smooth trial execution. This responsibility falls under the project management team.
After mapping out trial assumptions such as number of sites, patients, enrollment period, monitoring, a project manager will develop the study budget and timeline with key milestones.
Depending on the size and reporting structure of the company, project resources are either managed by the project manager or by functional managers.
Clinical project manager is able to connect the dots
A great project manager is able to connect two seemingly unrelated issues and assess the impact on the project.
Let me give you an example.
Say you’re in the midst of securing FDA approval to start a new clinical trial. You find out that the FDA has follow-up questions. This is going to delay the First Patient In (FPI) date. Your investigational devices with limited shelf-life are ready to be shipped to the sites.
A great PM will recognize the downstream effects of study startup delays. For example, she’ll start planning for the additional devices. Not only that but the PM will also adjust the study budget.
Clinical project manager anticipates issues
In addition, to be able to connect the dots, a project manager also anticipates issues.
For instance, a clinical site may be enrolling at a rapid pace. During a periodic data review meeting, the project manager finds out that the site has a significant number of protocol deviations.
Rather than letting the clinical site continue enrollment, the PM decides to put the site enrollment on hold until the compliance issues are fully addressed and resolved.
The PM anticipates the negative impact of compliance issues on the overall trial. If the PM does not anticipate the issue, it could have negative consequences on the final results and major audit findings for both the sponsor and the clinical site.
Clinical project manager can visualize and paint the big picture
A clinical trial has many moving parts. The objective of most trials is secure product approval, indication approval or assess the long term safety of the product after it is approved.
Keeping this end goal in mind, a clinical project manager needs to have a thorough understanding of the key milestones. If challenges arise (which they will), the project manager needs to communicate the challenges effectively to the right stakeholders.
It is also not uncommon for a project manager to paint the big picture for the team. This is necessary to prevent the team from digressing on irrelevant topics or getting distracted.
Clinical project manager is not afraid to get his/her hands dirty
When I started my career, I felt that the project manager’s primary job was to tell other people what to do and by when it needs to be done.
That certainly did not turn out to be true.
Most of us desire a “perfect” project with no issues or challenges. This is however not reality.
There will be obstacles along the way that a project manager will need to overcome.
For example, let’s consider a clinical site that urgently needs investigational devices to enroll a patient the next morning. Unfortunately, the FedEx package drop-off time is 4:00 pm and the pick-up truck has left for the day. The only way to get the device to the site in time for the procedure is to drop off the device package to the airport. A great project manager would drive to the airport and drop off that package.
Operations may be further subdivided in Internal Operations and External Operations. Members of the operations team usually have titles such as clinical research associate or clinical research manager.
CRA champions site start-up
The in-house operations team is primarily responsible for clinical trial site start-up. This team ensures that each site has the most recent version of the site start-up packet. The start-up packet consists of the protocol, ICF template, and other study specific documents.
The in-house operations team also facilitates clinical contract negotiation with the site, which includes the study budget.
CRA understands devil is in the details
Once the study-specific ICF template is sent to the sites, the site’s personnel review and edit the ICF to meet their site-specific IRB/ Ethics Committee requirements. The redlined ICF is then sent to the in-house operations team for final review and approval.
Similar to the ICF redlines, the CRAs also receive redlines on the clinical contracts.
This means that in-house operation is constantly inundated with multiple documents and redlines. This requires the in-house team to be detailed oriented and organized. An error can have significant ramifications including potential audit findings or lawsuits.
CRA’s life revolves site management and support
The in-house operations team is available for site management and support. Aside from ICF and clinical contract questions, it is usual for sites to contact their in-house CRA with questions about clinical protocol, study specific requirements, investigational device restocking, and more.
Field CRAs are also known as monitors.
In-house operations and field operations roles can sometimes overlap. In some organizations, field and in-house operations teams are combined into one group, namely Clinical Operations.
CRA is the face of the study for clinical sites
A field team is the “face of a study” for a clinical site. Most field operation team members work remotely from their home office. They travel to clinical sites on a regular basis for monitoring and site support.
The field CRA is also responsible for conducting pre-study visits, site initiation visits and study close-out visits.
CRA understands devil is in the details
A field CRA is responsible for monitoring clinical trial data at the site.
A field CRA needs to have thorough understanding of the study protocol, Good Clinical Practice (GCP) and regulations applicable to the conduct of clinical trials.
A clinical trial database has numerous fields. Depending on the monitoring plan, a CRA needs to review and verify the accuracy of the source data at the site.
For example, the CRA ensures the patient informed consent was signed prior to the procedure, serious adverse events were reported on time or protocol deviations were addressed appropriately. This work requires attention to details and deep focus to be done right.
CRA helps site succeed on all fronts – start-up, enrollment, compliance
Site coordinators can get overwhelmed with multiple trials. Many clinical sites are also nonprofit organization with limited funding and resources to support clinical research.
A field CRA can help their clinical site be successful by promptly answering all questions during study start-up, enrollment and follow-up.
In addition, it’s not uncommon for a CRA to prep sites prior to any scheduled audits.
The job of the field CRA is not “police” the site but rather be the site’s champion and help a site be successful in their research efforts.
Products are brought to market based on clinical data. Government agencies, medical community, and patients believe in the power of data.
It is no easy task to collect and clean hundreds of clinical trial data points in a compliant manner. This is where the data management team comes to play.
Data manager decides how data will be collected and cleaned
One of the key responsibilities of a data manager is to develop case report forms (CRF) for the collection of clinical trial data. These days most data is collected via electronic data capture (EDC) forms.
A well-designed and thought out CRF can be of great value to the sponsor in the long run.
For example, data collected in a case report form can not only help secure product or indication approvals but also forms the basis of publications and presentations.
Data manager’s life revolves around database locks
Once all patients have completed their primary endpoint visit and the data has been cleaned, the final step is to lock the database.
Database lock is a very important milestone not only for data management but also for the entire organization.
Database locks can be a very stressful time for data managers, sites and CRAs. The CRAs are frantically working with the clinical sites to resolve open queries. A database is usually not locked till open queries are resolved.
Once the database lock occurs, the biostatistics is able to analyze the data and generate tables and graphs.
Data manager communicates study metrics
Without data metrics, you would have no way of knowing whether your sites are completing the CRFs in a timely manner.
Let’s consider a 1000 patient clinical study at 65 sites with 3-year patient follow-up. For each patient there are 20-30 case report forms with multiple fields in each form. It becomes increasingly complex to determine what data is missing, incorrectly entered or not available.
A data management team can create custom reports that can provide data metrics by site, by visit or by patient. This information makes it efficient for sites and CRAs to address data entry gaps and query resolution.
Biostatistics (or Biometrics)
Statisticians are the numerical brain behind a clinical study. Statistics is a very broad field with numerous data analysis methods.
Biostatistician is the key driver behind trial design
One of the key components of clinical trial design is the sample size. Simply speaking, you want to know how many patients are needed in order for the clinical study to be statistically sound.
For many studies, regulatory agencies review the statistical analysis plan (SAP) as part of the trial design review.
Life revolves around p-values, trial power and performance goals
P-values, power and performance goals are the geeky pieces of information that statisticians care about.
Statisticians want to understand if the trial results (good or bad) are replicable in the real world.
They attempt to understand the probability of the certain benefits or risks to re-occur in real world once the medical products are commercially available.
Shares clinical study results using tables and graphs
The Statistical Analysis Plan (SAP) specifies how the data will be analyzed.
Once the clinical data is analyzed, it is presented beautifully in the form of tables and graphs. This is a key task as it forms the foundation of how the trial results will be communicated to the outside world.
Tables are graphs utilized in clinical summary reports, annual updates, presentations and publications. Tables and graphs are also included on product Information For Use (IFU) documents and Patient Guides.
When the product is approved to be sold commercially, sales and marketing teams use government approved tables and graphs to promote the use of the drug or device.
Clinical Safety
This is a very interesting role for anyone who wants to be closest to the medical aspects of any clinical trial.
Safety touches most aspects of a clinical trial including the protocol, patient informed consent, patient safety outcomes in the final study report, Instructions for Use (IFU) document and more.
Understands regulatory requirements around patient safety
Regulatory agencies are most concerned about the safety of the clinical trial procedure and investigational device or drug.
The safety team has a thorough understanding of regulatory requirements pertaining to the safety of any clinical trial.
When patients are enrolled in a clinical trial, they may experience an adverse event. Clinical sites, sponsors and CROs are required to meet strict adverse event reporting requirements. Adverse event reporting requirements can vary by country.
A safety team member has a clear understanding of regulatory requirements and helps ensure safety compliance.
Life revolves around adverse events management
A safety monitor champions all safety aspects of a clinical trial such as adverse events, clinical trial procedure risks, and device/ drug risks.
When a patient participates in a clinical trial, he or she may experience adverse events, also known as AEs. These AEs are reviewed by the safety team.
In some cases, there may be unexpected serious adverse events (SAEs) that may impact patient safety. When such events occur, the safety team evaluates and communicates the adverse event information with stakeholders such as clinical trial sites, patients, and regulatory agencies.
Manages Clinical Events Committee (CEC) and Data Monitoring Committee (DMC)
When patients experience AEs, the clinical sites reports the AEs to the trial sponsor or CRO. The safety team reviews reported AEs and collects relevant medical records.
A subset of these AEs and the corresponding relevant medical records are sent to a physician committee, known as the Clinical Events Committee (CEC). The safety team manages the selection and operation of a CEC.
The basic premise of a clinical trial is that it’s a drug or medical device experiment on human beings. Participation in a clinical trial usually involves safety risks. Some safety risks are anticipated and others aren’t.
For unanticipated risks during the enrollment phase, a sponsor may recruit a data safety monitoring board (DMC). The DMC ensures there is no risk to patients in the trial.
Not all trials require DMC as the duration of enrollment might be too small to detect a safety signal. Similar to CEC, the safety team leads the selection and management of a DMC.
Clinical Quality
Throughout my career, I’ve worked with clinical quality experts that either “police” every step in the clinical process or serve as a partner and resource to other clinical research functions.
In both cases, the ultimate goal of any clinical quality personnel is to keep you, your clinical trial and your organization out of trouble.
Ensures compliance in all aspects of a clinical trial
As we’ve discussed earlier, clinical research is a highly regulated industry. With regulations, comes compliance. Clinical research professionals need to comply with government regulations, Good Clinical Practice (GCP) and operating procedures.
You may ask, “What’s the purpose of all this compliance”?
Well, the simple answer is that the outcome of a clinical trial sets a new medical standard or leads to an update of an existing medical standard.
Would you trust a drug or device that is brought to market based on a non-compliant clinical trial?
Probably not.
Clinical quality wants no audit Findings
Passing an audit is the ultimate test of any clinical research organization. This is especially true for audits by regulatory agencies.
Audit findings are painful not just for the quality team but for the entire clinical team. Significant audit findings also raise doubts about the robustness of a clinical trial.
Life revolves around Standard Operating Procedures (SOPs)
For instance, in the US, FDA regulations dictate how a clinical trial should be conducted. These regulations form the basis of standard operating procedures (SOPs).
A great quality associate helps a clinical research organization create and maintain a practical set of SOPs.
It is important to note that the burden of creating SOPs does not lie with clinical quality. Other clinical research functions are responsible for drafting and finalizing their own role-specific SOPs.
Understands Corrective and Preventive Action Plan (CAPA)
Since many aspects of clinical trials are managed by humans, the process is prone to errors. Errors can happen due to oversight, gap in a SOP, or mismanagement. A quality personnel initiates a CAPA when errors are discovered.
The goal of a CAPA is to document the error and take necessary steps prevent the error from happening in the future.
CAPAs are frowned up and are never a good thing for any organization. More CAPAs means there is greater risk, which in turn reduces the public’s confidence in the clinical trial outcomes, product or company.
Medical Writing
Clinical trial information is communicated with all stakeholders via clinical documents such as the protocol, clinical reports and manuscripts.
A medical writer leads the task for assimilating trial information and putting it together in a logical way.
Understands scientific content
A medical writer has an in-depth understanding of the therapeutic area and previous clinical and preclinical information on the drug or device.
Given the countless number of medical products and trials, it’s not unusual to learn trial or product specific information after joining the project.
Life revolves around writing protocols, reports or manuscripts
As a medical writer, you need to enjoy the process of writing. If you don’t enjoy writing, it will be painful to write several hundred pages of clinical trial documents on a regular basis.
A medical writer has a unique ability to communicate high-level clinical trial strategy vision in a document such a protocol or report.
Regulatory decisions are made based on these documents. Therefore it is extremely important for a writer to understand what regulators are looking for.
Detailed oriented with focus on punctuation, grammar and formatting
A medical writer is responsible for writing key clinical documents such as the protocol, clinical reports, safety charters, and more.
Punctuation, grammatical errors or typos can reduce the reviewers trust in the final document. Such errors make the company look sloppy in front of regulators, clinical sites or trial sponsor.
Additionally, a medical writer needs to master writing software such as Microsoft Word. Inability to effectively use such software will cause issues with document formatting, resulting in delays and frustration.
Medical Science
A scientist is responsible for the clinical trial strategy.
In some organizations, the role of the scientist may be combined with that of the medical writer or that of project management.
Brainchild behind the overall clinical strategy
Prior to the start of any clinical trial, a lot of work is put into designing a cost-effective, safe and effective study.
Depending on the trial design, there may be quite a bit of back-and-forth between the regulator and the sponsor or CRO.
A clinical trial can begin enrolling patients only after the regulatory alignment is obtained.
The scientist champions the development of clinical documents and communication necessary to secure this regulatory alignment.
Life revolves around designing a trial that meets the primary endpoint
If you remember your life as a student (or maybe you’re still a student), your final grade on the test depends on how well you do on the finals.
Along the same lines, the success of any clinical trial is determined on whether or not it meets a pre-specified primary endpoint.
The trial endpoints are clearly stated in the protocol. Once the primary endpoint is met, the medical product will most likely be approved for commercialization.
Medical science wants high-quality clinical trial data
The success of a clinical trial is hinged on high-quality clinical trial data. But what does “high quality” mean?
High-quality data is error-free, accurate, and complete. Missing or incomplete data due to missed visits or sloppy data entry can cause headaches for many clinical stakeholders, primarily the clinical scientist.
Also, there can be a tendency to collect more data than needed for “just in case” scenarios such as unanticipated requests from regulators or potential for interesting publications in future years.
Clinical Systems and Solutions
Not too long ago, clinical trial data was collected on paper case report forms. Then it became incredibly hard to manage data queries, keep track of complete forms and manual entry of data from paper into an electronic database.
With the help of technology, electronic data capture (EDC) solutions were developed. Now site staff can directly enter data in the case report forms.
The Systems and Solutions team is responsible for managing clinical technology solutions such as the EDC. They are also responsible for onboarding new technology solutions and retiring old solutions that no longer add value to the organization.
Technology backbone for clinical trials
More than ever before, technology is becoming an integral part of clinical research. The system and solutions group is the technology backbone for clinical trials.
Want to incorporate wearable technology in a clinical study?
Or build an iPhone app to organize study team contact?
Clinical systems and solutions group is your go-to team.
Depending on the size of the company, this role may be a separate function within clinical research or part of the information technology (IT) organization.
Focused on clinical research software and applications
Clinical research teams utilize a few core technology solutions such as the Trial Master File (TMF), Clinical Trial Management System (CTMS), Electronic Data Capture (EDC), Integrated Web/Voice Response System (IxRS), and electronic Informed Consent (eConsent).
The TMF allows you to store study and site level documents in electronic format.
CTMS is useful for managing study contact information, monitoring trip reports, site and vendor payments and more.
EDC allows sponsors to design electronic case report forms and allows sites to remotely enter data into the system.
IxRS serves to register patients to a specific treatment.
In addition, there may be other systems for safety management and reporting, protocol deviation and monitoring visit management.
Understands regulatory requirements for technology solutions
Regulators are interested in ensuring clinical trial technology is compliant with the law.
At the most basic level, technology solutions need to maintain an audit trail, protect patient personal health information (PHI) and encrypt sensitive clinical trial information.
Systems and solutions teams need to have an in-depth understanding of regulatory requirements pertaining to technology solutions.
Regulators such as the FDA frequently publish guidance documents related to a specific technology product.
Guidance documents can be easily accessed via the FDA website and can serve as a great resource for anyone wanting to dig deep in a specific clinical technology area.
Senior Management
Senior management is ultimately accountable for the success (or failure) of any clinical trial.
Key senior management functions in a clinical research organization include directors of each of the functional teams described above.
Depending on how the organization is structured, a clinical director may oversee more than one clinical trial or clinical function.
Accountable for all aspects of a clinical program
Providing adequate oversight is the key role of a senior manager.
Oversight is not the same thing as “micro managing” an employee or a task. For certain critical deliverables, a senior manager may be more hands-on.
Strategic focus – Budget, Timelines, Resource Allocation, Procedure Compliance, Scientific Robustness
Clinical trial costs can run into millions of dollars. By controlling project timelines and resources, a senior manager can control expenses.
In addition, it is crucial to ensure the trial is conducted in compliance with regulations and internal procedures.
Finally, if the study is not scientifically robust, the clinical trial may not get regulatory approval.
It is not possible for one individual to be competent in all these areas. For this reason, a senior management team is needed to collaboratively achieve strategic goals.
Interested in Key Performance Indicators (KPIs)
Do you have a retirement account?
Or go to the doctor for an annual check-up?
If so, you’re probably paying attention to the performance of your retirement fund or your health over a period of time.
Along the same lines, senior managers are interested in a set of KPIs that track the performance of a clinical trial against a standard or threshold.
For example, regulators want to ensure patients sign the correct version of the informed consent form (ICF). Signing an incorrect ICF version is a major compliance issue. Thus ICF deviation rate can be a KPI.
Since senior managers are not involved in the day-to-day operations of the trial, they assess the health of a project by reviewing KPI metrics. If KPI metrics trend towards non-compliance, senior managers will take steps to address the issues.
Other Clinical Trial Stakeholders
A clinical department frequently collaborates and seeks help from other teams. The section below covers the key teams that interact and support clinical teams on a regular basis.
Since the focus of this post is clinical research, the role of these adjacent functions has been described keeping in mind the clinical context.
Regulatory Affairs (RA)
Regulatory Affairs (RA) is responsible for regulatory strategy and submissions. For a clinical trial, a RA team member is generally the primary contact person for the regulator such as the US FDA, Japan PMDA, China FDA.
Deep understanding of regulatory process and requirements
Ready to start a clinical trial? Or address clinical trial deficiencies with the regulator?
You’ll want to start with your regulatory affairs team. Clinical trials are conducted in compliance with regulations. RA can educate you on the clinical documents needed to meet regulatory requirements in a given geography.
A regulatory affairs expert has an in-depth understanding of the regulatory process and requirements.
Adept at reviewing and editing clinical submission documents
Once the clinical team has created the necessary documents such as the protocol, statistical analysis plan or the clinical summary report, a regulatory affairs associate performs a detailed review of the documents.
In addition, regulators have specific formatting requirements such as cover letters and forms that need to be completed with every submission.
Focus is on regulatory agency alignment on all aspects of clinical research
There is a specific way of communicating clinical trial information with regulators. This communication style is very different from the day-to-day communication amongst clinical research team members. A seasoned regulatory affairs expert understands this nuance and is able to communicate effectively with regulators.
Seeks new medical product/indication approvals
Success of a regulatory affairs team is measured by product and indication approvals. It’s no easy task to get a product to market, especially for new therapies.
Health Economics and Outcomes Research (HEOR)
Getting medical products or indications approvals is always great news.
But here’s the deal.
You need someone to pay for the approved drug or device. If payors such as insurance companies or Medicare are not willing to provide the reimbursement, it can significantly hurt a company’s bottom line.
This is where HEOR comes into play. A HEOR team helps create a body of evidence that the new drug or device has health benefits such as improved quality of life.
Deep understanding of payor process and requirements
A HEOR expert can help you develop a robust protocol and case report, so you can proactively collect the clinical data needed for reimbursement.
HEOR has an in-depth understanding of country-specific requirements. Their work leads to maximum medical product reimbursement for the company.
Understands medical product impact on Quality of Life (QoL)
Let’s say your friend experienced a heart attack and got a stent. Now 10 years have passed. Your friend has not experienced any new heart problems. He feels great, goes for a morning run and is able to spend quality time with his family.
How would you rate his quality of life? Excellent, right?
HEOR pays much attention to a patient’s quality of life. In addition to being safe and effective, medical products need to have a positive impact on a patient’s quality of life.
There are many tools available to measure such quality of life. Clinical trials include quality of life questionnaires for patients to complete. This data is then analyzed and utilized to secure reimbursement approvals.
Seeks to maximize medical product reimbursement
One of the primary roles of the HEOR team is to ensure the medical product receives maximum reimbursement possible.
Reimbursement can vary significantly based on country laws or availability of new health data. For example, Japan offers higher reimbursement for newer medical products but then decreases reimbursement in future years.
Clinical Research Organizations (CROs) and Consultants
Some sponsors hire full-time staff to manage their trials. Some outsource all clinical research tasks to CROs and consultants. While others may use a combination of internal and external resources.
Supports sponsor by filling in resource and talent gaps
It is expensive to build a full clinical team, especially for an organization with limited financial resources. CROs can help fill the talent gap for each of the clinical research roles discussed in this post.
Work is billable
A major chunk of clinical trial costs is full-time salaries paid for each of the clinical roles explained in this post.
CROs and consultants charge by the hour or by deliverable. The “pay by the hour” or “pay by deliverable” model gives sponsors greater flexibility and control on expenses.
People that work for a CRO may be full time employees or contractors. At any given time, a CRO or a consultant may be working on multiple projects for different sponsors.
Can help reduce fixed overhead expenses
For smaller organizations, steady cash flow can be an issue. Therefore having a full clinical team means paying for employee salaries, office space, health insurance and other benefits. This can be cost prohibitive.
Hiring a CRO or consultant can reduce fixed expenses, giving companies greater flexibility with their limited resources.
Legal
Clinical research cannot be conducted without legal support.
You’d open yourself to all sorts of risks if you didn’t have a lawyer on your team.
Complete understanding of legal landscape
A lawyer with clinical research experience has an understanding of intellectual property law (patents, trademarks), legal entities i.e. how the hospitals or suppliers are structured from legal standpoint, supplier contracts, subject injury and liabilities, and more.
Seeks legal compliance in a clinical trial
A lawyer will be extremely happy if a company is successfully able to conduct a clinical trial in a legally compliant manner.
For instance, there is great risk to patients participating in clinical trials. If the legal language on the informed consent form or clinical trial contract between the site and sponsor is inadequate or unclear, it can lead to lawsuits.
Lawsuits impact the company’s finances, brand and reputation. A legal team ensures the company does not end up in legal troubles with any of the clinical trial stakeholders.
Conclusion
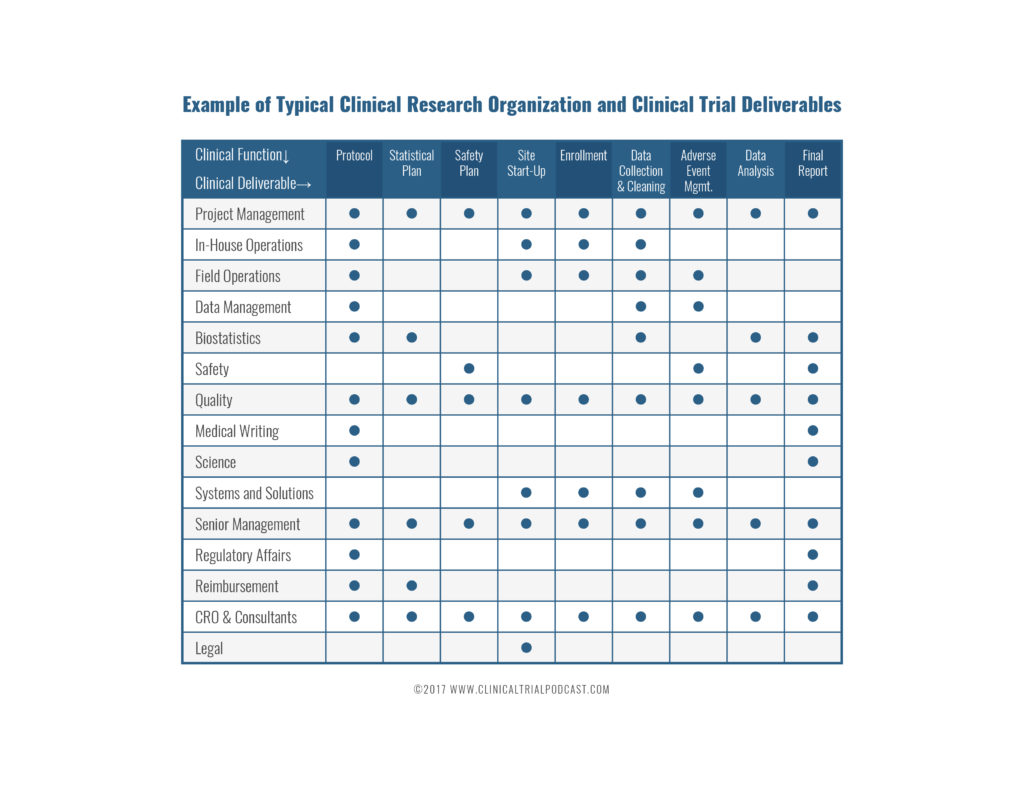
By now you have an in-depth understanding of all clinical research functions and teams that support clinical trials.
Clinical research and clinical trial management involve cross-functional teams. The chart below summarizes the roles of different clinical research team members.
Which clinical research function interests you the most? Leave your response in the comments section below.


25 Thoughts on "Who’s Who in Clinical Research – A Complete Guide (2022)"
Ultimate Guide to Clinical Trial Costs - Clinical Trial Podcast
25 Soft Skills To Boost Your Clinical Research Career – Clinical Trial Podcast
10 Smart Strategies to Getting Response from Clinical Site Coordinators – Clinical Trial Podcast
5 Ways To Get Clinical Research Associate Experience – Clinical Trial Podcast
5 Ways To Engage Clinical Trial Sites With Technology | Florence Healthcare
Conversation with Clinical Research Expert | Marshall Cool
How To Get A Clinical Research Job | Clinical Trial Podcast
How To Get Clinical Research Associate (CRA) Experience – Clinical Trial Podcast
how to become a cra – CAREER KEG
9 Essential Components of a Clinical Trial Agreement - Clinical Trials Arena
Sonia Menezes
July 5, 2017I have a masters in clinical research and work experience at an oncology site. I’m looking to get into to industry and medical writing is the most interesting to me. I wanted opinions on what people in the industry think about the field and whether it is too niche to start your with?
Kunal Sampat
July 5, 2017Hi Sonia, I would suggest starting as a medical writer in clinical research. There is a huge demand for great medical writers. There are several medical writing groups on LinkedIn that you may want to consider joining. Reach to people in those groups and find out how you can get started. It’s not a small niche and the opportunities are endless. You can always move in different directions as long as you remain curious and interested in other roles. Goodluck!
mamtha srinivas
December 6, 2017hi I have worked in clinicalresearch for last 4 years. i have an opportunity to work under investigator directly .I need to search for new projects.Guide me how to go about consulting for projects.
Kunal Sampat
December 21, 2017Hi Mamtha, I would recommend you follow the steps outlined in the blog post. https://clinicaltrialpodcast.com/get-a-clinical-research-job/. Let me know if you have any additional questions. Goodluck!
Subuloye Olufunke
February 1, 2018Hi,
Please for someone thinking of going into the clinical research field with no experience, would you suggest getting an online training or getting a certification in one of the Universities? Which one has a better chance of getting a job considering the fact that there is no experience. Thanks.
ihanshi
February 14, 2020An informative read. As an clinical research novice it imparts more knowledge about the field.
Kunal Sampat
April 22, 2020great, thanks for the feedback
Gabriel
March 13, 2021Hi,
Please for someone thinking of going into the clinical research field with no experience, would you suggest getting an online training or getting a certification in one of the Universities? Which one has a better chance of getting a job considering the fact that there is no experience. Thanks.
Fran Ross
April 13, 2021Kunal, you’ve missed the Risk Manager, a critical role per ICH E6 R2, and expect even more important in upcoming R3.
Love your work!!!
Kunal Sampat
April 24, 2021Thank you, Fran for your thoughtful comment. Safety monitors and quality associates can also serve as Risk Managers
Jane T
April 23, 2021This is such an informative post. I’m a Registered Dietitian and another RD informed me that RDs make good Project Managers in research; she works as one. I came across this post when looking for the “job ladder” and responsibilities involved in the field and its exactly what I needed. Thank you for compiling it!
raveena aher
July 28, 2021Hey thanks for sharing this blog over here. It seems useful to start career in clinical research. We will look forward for more updates.
Rahul
August 18, 2021It is a pleasure to hear you share such useful information.
Moses Musitwa
February 5, 2023Safety monitors and quality associates is some fascinating role,as a medical doctor, medical educator and manager which role would suite me because am spoiled for choice.
Kunal Sampat
June 29, 2023Safety monitors and quality associates are fantastic clinical research positions for doctors to pursue.